Temperature Controlled Programmed Lab Services
Temperature-programmed analyses are used to study chemisorption bonds under controlled conditions of varying thermal energy.
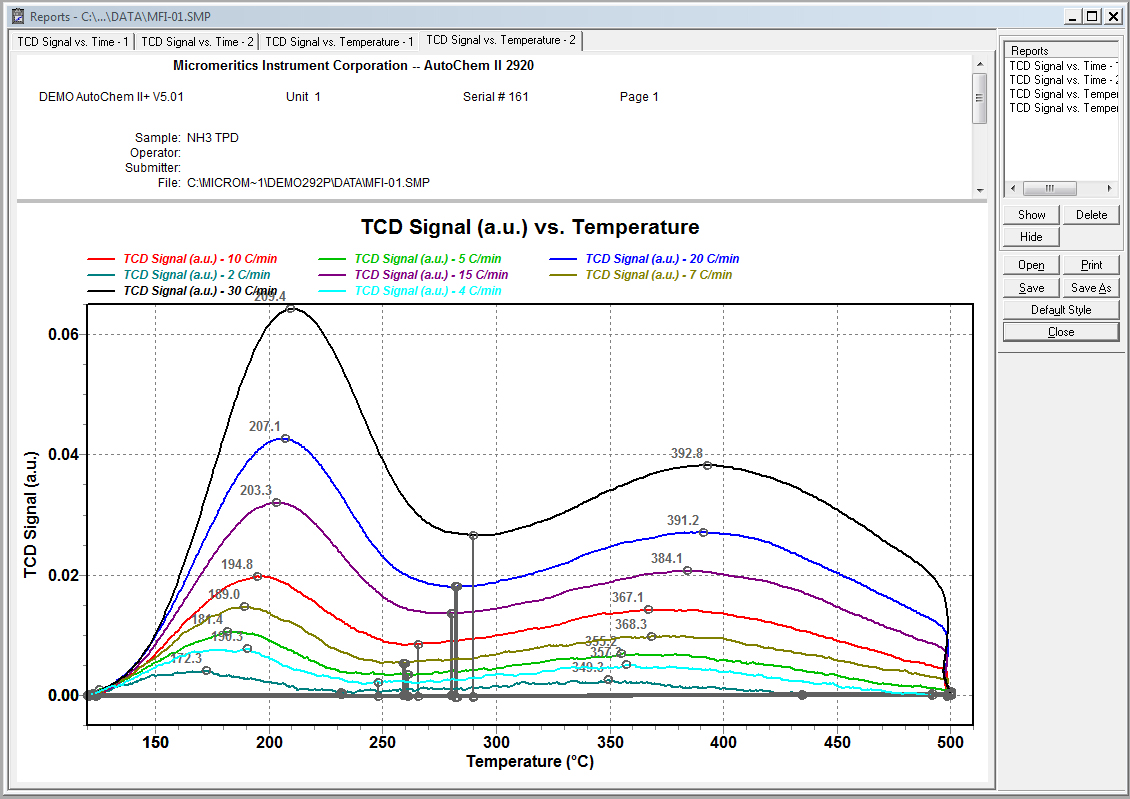
TPD- Temperature Programmed Desorption
TPD is used to study the desorption of physical and chemically bound species on the surface of a material. the temperature of the sample is increased until the thermal energy is sufficient to break the chemisorption bond. By monitoring the temperatures at which molecules are liberated and determining the volume of gas desorbed at each temperature, the strength and number of adsorption sites can be determined.
TPR- Temperature Programmed Reduction
TPR (reduction) is used to measure the quantity of reducible species in the sample. This analysis is typically used to evaluate metal-supported catalyst. while
TPO- Temperature Programmed Oxidation
TPO (oxidation) is used to quantify the oxidizable sites contained within a material. More commonly,
TPO is used in applications such as the study of the kinetics of coking, evaluation of catalyst carbon burn-off, determination of the different forms of carbonaceous deposits present on the catalysts after a CO decomposition reaction or, stated more generally, measurements of oxygen consumption and product yields.