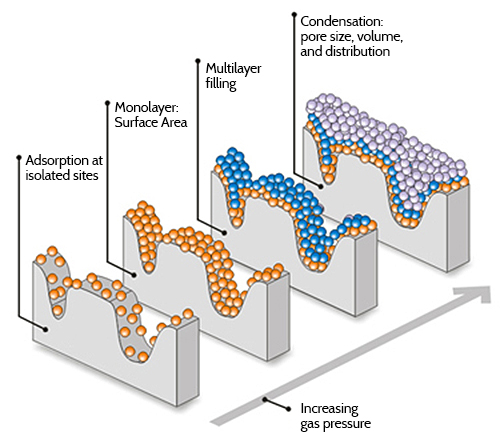
Gas Adsorption Technique
To measure pore size by gas adsorption, isotherms (typically using N2, Ar, or CO2) are recorded from low pressures (approximately 0.00001 torr, minimum) to saturation pressure (approximately 760 torr). The pressure range is determined by the size range of the pores to be measured. Isotherms of microporous materials are measured over a pressure range of approximately 0.00001 torr to 0.1 torr. Isotherms of mesoporous materials are typically measured over a pressure range of 1 torr to approximately 760 torr. Overall, gas adsorption is applicable to pores ranging from 3.5 Angstroms to about 4000 Angstroms in diameter.
Once details of the isotherm curve are accurately expressed as a series of pressure vs. quantity adsorbed data pairs, a number of different methods (theories or models) can be applied to determine the pore size distribution. Available micropore methods include: Density Functional Theory (DFT), MP-Method, Dubinin Plots (Dubinin-Radushkevich D-R, Dubinin-Astakov D-A), and Horvath-Kawazoe (H-K) calculations. Available Mesopore methods include: Barrett, Joyner and Halenda method (BJH), and Density Functional Theory (DFT). T-Plot analysis is also available for total micropore area as well.